· What is the Bohr model of potassium?1500 · The Bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific distances from the nucleus (Figure 1) These orbits form electron shells or energy levels, which are a waySo in the last video I showed you how to get this equation using a lot of physics and so it's actually not necessary to watch the previous video you can just start with this video if you want and E one we said was the energy associated with an electron and the lowest energy level of hydrogen and we're using the Bohr model and we calculated the value for that energy to be equal to negative
Arrangement Of Electrons
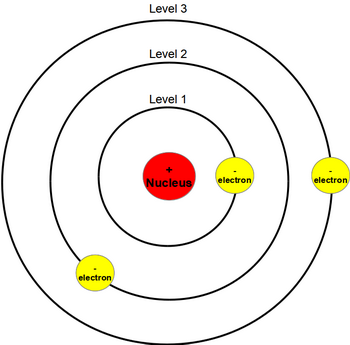
Bohr model of atom diagram
Bohr model of atom diagram-Niels Bohr modified Rutherford's Nuclear Atom model to explain how light interacted with the electrons in an atom to produce spectral lines His model included electrons orbiting the nucleus at specific energy levelsBohr model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the Danish physicist Niels Bohr The Bohr model of the atom, a radical departure from earlier, classical descriptions, was the first that incorporated quantum theory and was the predecessor of wholly quantummechanical models The Bohr model and all of its successors describe the properties of atomic electrons in terms of a set of allowed (possible) values Atoms




Models Of The Atom The Atom Siyavula
· Bohr's Model of the Atom In 1913, Neil Bohr proposed a theory to explain the stability of an atom, where electrons revolved around the nucleus at certain energy levels Each orbital had a different radius and electrons revolving in a particular orbit did not radiate energy This happened even if the electron had accelerated motion around the nucleus Bohr put forth some postulates that supported his atomFriends We are working hard for more Educational videos for You and Learners Please Subscribe to our channel and click the bell icon If you like this videoToday the atomic model is very similar to Bohr's model The modern atomic model, the Wave model, does not have exact orbits like Bohr did Instead the modern model has a scatter region surrounding the _____ where an _____ will probably, but not certainly, be found The Atomic Model of Matter Key More than 00 thousand years ago, A Greek philosopher namedDemocritus led a
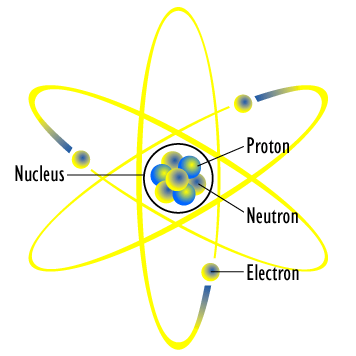
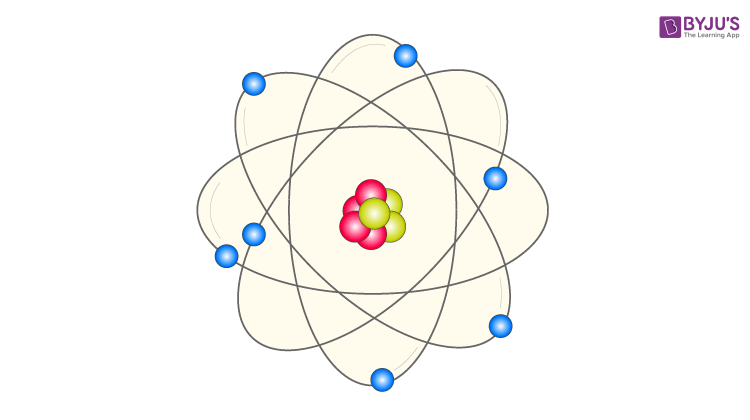
Bohr's model of the atom (ESAAR) There were, however, some problems with Rutherford's model for example it could not explain the very interesting observation that atoms only emit light at certain wavelengths or frequencies Niels Bohr solved this problem by proposing that the electrons could only orbit the nucleus in certain special orbits at different energy levels around the nucleusBohr's Model of the Atom •Focuses on electrons and their arrangement •Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun •Bohr correctly assigned energy levels to electrons, but electrons do not move in fixed orbits around the nucleus Bohr's Model of the Atom Electron Nucleus Valence Electron Electron Cloud ModelHe hypothesizes that the angular momentum of an electron in orbit around a proton is quantized (ie it can only be a discrete multiple of a certain number) Under this simple assumption he managed to compute the energy of the
A Danish physicist named Neil Bohr in 1913 proposed the Bohr atomic model He modified the problems and limitations associated with Rutherford's model of an atom Earlier in Rutherford Model, Rutherford explained in an atom a nucleus is positively charged and is surrounded by electrons (negatively charged particles)Bohr's model for H atom In 1913 Neils Bohr proposed a physical model to describe the spectrum of the hydrogen atom It was the birth of Quantum Mechanics!3003 · To draw a Bohr model of an atom, first find the number of protons, neutrons and electrons in the atom from its atomic weight and atomic number After that, place the neutrons and the protons in the nucleus, and draw the electrons in their designated shells Find the number of electrons, protons and neutrons
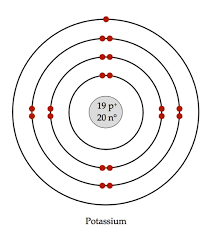



What Is The Bohr Model Of Potassium Socratic



Bohr Model Wikipedia
· Bohr Model of Phosphorus Neon Atom Model, 5th Grade Science Projects, 8th copper bohr diagram wedocable 28 images copper element protons and As was mentioned before, a neutral Phosphorus Atom contains five valence electrons in the third shell You can see a Bohr Model of Phosphorus below this Follow these steps to draw a Bohr model for phosphorus Use whichever style of Bohr modelThe Bohr model of the hydrogen atom explains the connection between the quantization of photons and the quantized emission from atoms Bohr described the hydrogen atom in terms of an electron moving in a circular orbit about a nucleus He postulated that the electron was restricted to certain orbits characterized by discrete energies Transitions between these allowed orbits result2 Use the table above to draw the Bohr model diagram for the following atoms and ions Argon atom 22N Chlorine atom Chlorine ion 19 Potassium atom Potassium ion 3 What do you notice about the arrangement of electrons in the Bohr model of a neon atom, fluorine ion, and a magnesium ion?
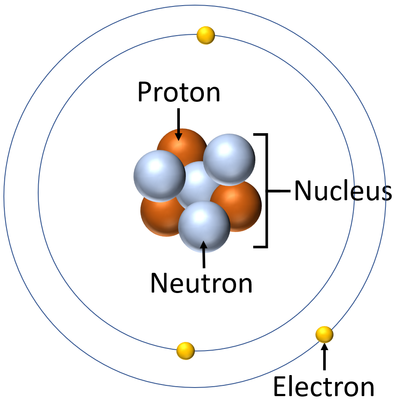



Atom Key Stage Wiki




How To Draw Bohr Diagrams Slideshare
· Since Bohr's model involved only a single electron, it could also be applied to the single electron ions He , Li 2, Be 3, and so forth, which differ from hydrogen only in their nuclear charges, and so oneelectron atoms and ions are collectively referred to as hydrogenlike or hydrogenic atoms The energy expression for hydrogenlike atoms is a generalization of the hydrogen atomThe Bohr model was an advancement to an earlier atomic model, the Rutherford Model Key features of the Bohr model include set orbits for electrons around the nucleus, as well as the ability of electrons to move from one orbit to another The Bohr model was later superseded by the Sommerfield model · In the Bohr model, electrons are confined to concentric spheres around the nucleus numbered as n=1, 2, 3, The sphere n = 1 can accommodate two, the n = Model sulfur atoms are complex, containing nearly 50 parts the Bohr atom model with fixed electrons as a way to simplify atomic structure Sulfur (S) Diagram of the nuclear composition, electron configuration,
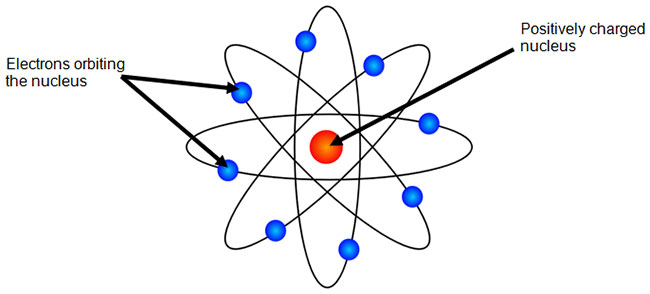



The Bohr Model Texas Gateway



Bohr Model Of The Atom Overview And Examples
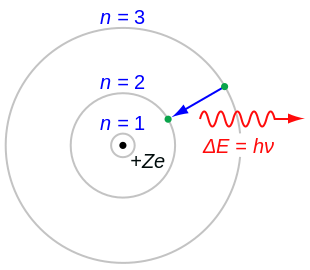
The orbits are labeled by an integer, the quantum number n In Bohr's model, radius an of the orbit n is given by the formula an = h2n2 ε 0 /π 2, where ε 0 is the electric constant As Bohr had noticed, the radius of the n = 1 orbit is approximately the same size as an atomDerivation of Bohr's Equations for the Oneelectron Atom Bohr set about to devise a model that would explain the observed line spectra of oneelectron atoms, such as H, He, Li2 The model Bohr used was based on Rutherford's conclusion from his gold foil experiments that the negative electrons in an atom are a great distance away from the positive charge in the nucleus Bohr · /captionThe most widely accepted atom model is that of Niels Bohr Bohr's model was first introduced in 1913 This model of the atom depicts a small, positively charged nucleus surrounded by



Bohr Diagram



Bohr S Model Of Hydrogen Article Khan Academy
5 Below is shown the PES spectrum of sulfur (atomic number = 16) a Write the full electron configuration of sulfur b Label each peak in the spectrum to show which subshell it represents (ie, 1s, 2s, etc) c On the spectrum, sketch in the relative locations and correct peak heights for the spectrum of aluminum (atomic number = 13) By · Bohr's model didn't solve all the atomic model problems It worked well for hydrogen atoms, but couldn't explain observations of heavier elements It also violates the Heisenberg Uncertainty Principle, one of the cornerstones of quantum mechanics, which states we can't know both the exact position and momentum of an electron Still, this principle wasn't postulated untilThe Bohr model of the boron atom has a nucleus in the center and two energy levels, also known as shells, or orbitals, around the outside Two electrons orbit the For example, a Bohr diagram of the element boron shows five protons and five electrons




Atom Diagram Universe Today



Bohr Model Of The Atom Overview And Examples
· When we talk concerning Label an Atom Worksheet, we've collected particular variation of photos to inform you more drawing atoms worksheet, blank bohr model worksheet and labeled parts of an atom diagram are three of main things we will show you based on the gallery title Beside that, we also come with more related ideas like label parts of an atom diagram, atomic structure of an atom worksheet and drawing atoms · Bohr model perfectly fits the hydrogen atom, which has a single electron and a small positively charged nucleus But there are few drawbacks of Bohr model when explaining the atomic structure of atoms other than Hydrogen Bohr model could not explain Zeeman effect (effect of magnetic field on the atomic spectrum) or stark effect (effect of electrical field on the atomic · Bohr's model of an atom 1)An atom is made up of three particleselectrons,protons and neutronsElectrons have negative charge,protons have 2)The protons and neutrons are located in a small nucleus at the centre of an atomDue to the presence of 3)The electrons revolve around the nucleus in



Bohr Model Diagrams Lesson 3 1 Extension Ppt Video Online Download



Bohr Model Of The Hydrogen Atom Equation Formula Limitations
Draw a neat, labelled energy level diagram for H atom showing the transitions Explain the series of spectral lines for H atom, whose fixed inner orbit numbers are 3 and 4 respectively Advertisement Remove all ads Solution Show Solution Paschen series i The spectral lines of this series correspond to the transition of an electron from some higher energy state to 3rd orbit ii ForBrowse 18 bohr atomic model stock photos and images available, or start a new search to explore more stock photos and images Etching of Arnold Sommerfield , the German physicist who modified Niel Bohr's model of the atom in 1916 He lectured for many years at the University Model of an hydrogen atom according to the Bohr theory of the · Key Terms Alpha Particles, Atom, Bohr Model, Electron, Line Spectra, Nucleus, Orbitals, Rutherford Model What is Rutherford Model Rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core It also states that the central core is positively




How To Draw The Bohr Rutherford Diagram For Calcium Youtube



Arrangement Of Electrons
The RutherfordBohr model of the atom is based on evidence obtained from alpha particle scattering experiments using the apparatus shown below Question Name the parts labelled A, · The model of the atom made by Neil Bohr depicts a positively charged nucleus surrounded by a negatively charged ring of electrons that travel in circular orbits It was a large advancement in the field because Bohr's model described, for the first time, that an electron must absorb or omit energy to move between orbits · In Bohr's model of a Hydrogen atom, electrons move in orbits labeled by the quantum number n Find the radius, in meters, of the orbit of an electron around a Hydrogen atom in the n = 5 state according to Bohr's theory Question In Bohr's model of a Hydrogen atom, electrons move in orbits labeled by the quantum number n Find the radius, in



What Are The Postulates Of Bohr S Model Of Hydrogen Atom What Is The Importance Of This Model To Explain The Various Series Of Line Spectra In Hydrogen Atom Quora



Q Tbn And9gctb9tau 2g7tvyjxpox Ev6bi9l0ie9qttj3tesrzvzjb1ahv8p Usqp Cau
The Bohr Model of the Atom Newton, the English physicist, hypothesized that light consisted of tiny particles and that a beam of light would therefore be a stream of particles Around the same time, Christian Huygens, a Dutch physicist, suggested that light traveled as a waveform in the same way energy travels in water Neither hypothesis became the dominant idea until 0 years later,Bohr Model Drawing Draw a Bohr model of a chlorine atom in the space below Be sure to place the electrons in the correct orbitals and to fill out the key for the subatomic particles Protons Neutrons Electrons Chlorine 3542 Atomic number equals the number of or Atomic mass equals the number of Identify the each of the parts of the box Oxygen · aluminum atom model Google Search 3d Atom Model, Atom Model Project, Bohr Model of Aluminum Atom Model Project, Bohr Model, Science Projects 13_aluminum_(Al)diagramwebnet ( × pixels, file size 23 KB, MIME type English 13 aluminum (Al) Bohr model with subshells Figure \(\PageIndex{2}\) contrast the Bohr diagrams for lithium, fluorine and aluminum atoms




The Structure Of An Atom Explained With A Labeled Diagram Science Struck




Bohr Model Description Development Britannica
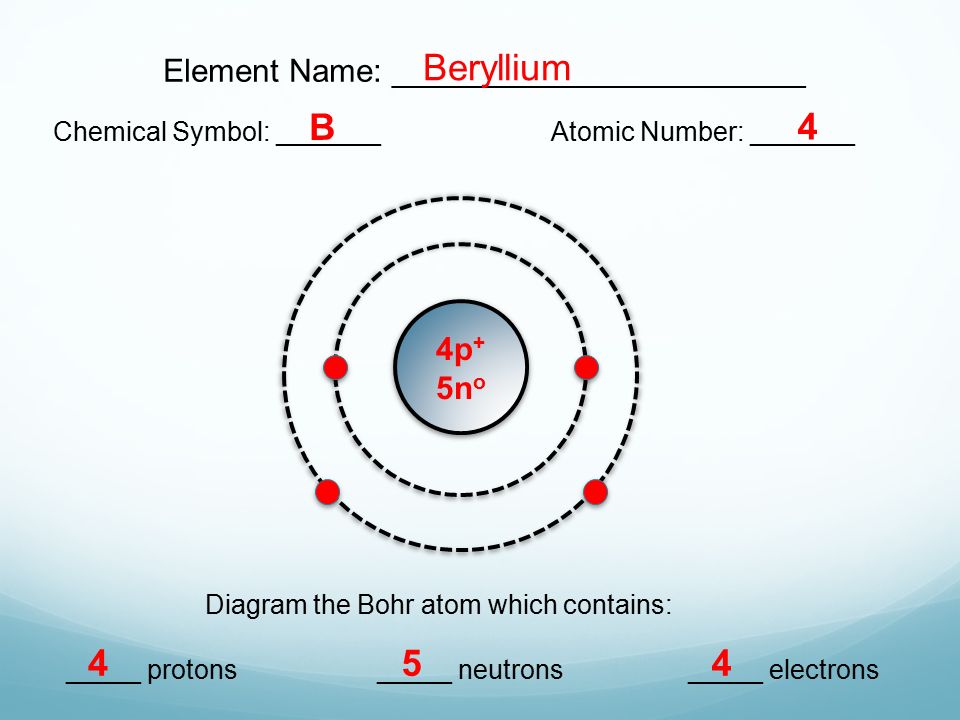
· Atomic physics Bohr model of the atom by crator Bohr And Quantum Mechanical Model of Atoms Bohr model and the quantum mechanical model This means the beryllium atom has four protons and four electrons The number Creating a 3D model provides a child with a visual representation of a beryllium atom0618 · 3 The number of neutrons is atomic mass atomic number Therefore, number of neutrons in nitrogen = 7 Bohr Model 1 Draw a small circle, inside that circle, write p = 7 and n = 7 The first outer shell holds 2 electrons So draw, 2 electrons on the circle Then draw the second shell, which can contain 8 electrons However, you only require 5Bohr Model of the hydrogen atom first proposed the planetary model, but later an assumption concerning the electrons was made The assumption was the quantization of the structure of atoms Bohr's proposed that electrons orbited the nucleus in specific orbits or shells with a fixed radius Only those shells with a radius provided by the equation below were allowed, and it was




Bohr Diagram Of Sodium Atom Diagram Atom Model Atom Model Project




How Atoms Work Atom Model Project Atom Model Bohr Model
How can I draw Bohr diagrams?Bohr Diagrams 1) Check your work 2) You should have 6 total electrons for Carbon 3) Only two electrons can fit in the 1st shell 4) The 2nd shell can hold up to 8 electrons 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons 6p 6nHow many electrons would it take to equal the mass of a helium nucleus?
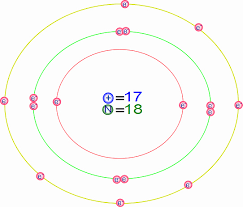



Is This Bohr Atomic Structure Of Cl Right Socratic




The Lab Lads Bohr Models
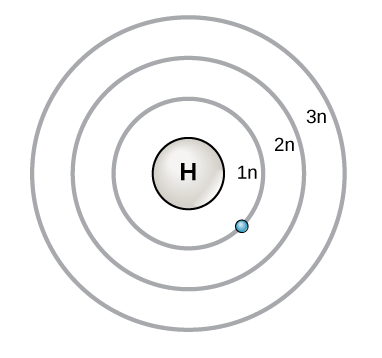
Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus Bohr's model calculated the following energies for an electron in the shell, Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where theThe Bohr model depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity Bohr's first postulate was that an electron in an atom could revolve in certain stable orbits without the emission of radiant energyChemistry Matter Basic Atomic Structure 1 Answer MathFactorialsblogspotcom May 31, 16 Please see the diagram below Explanation Answer link Related questions How big is an electron compared with a proton?




Dublin Schools Lesson Bohr S Model Of The Atom Whose Atomic Model First Accounted For Defined Energy Levels
.jpg)



The Bohr Atomic Model
ALI e ec Levels 4 What would you expect to see with the arrangement of electrons in the Bohr model



The Development Of The Atomic Model Wired



What Does Bohr S Model Of The Atom Look Like Socratic




In Bohr S Model Of The Atom Where Are The Electrons And Protons Located Homeworklib



Bohr S Atomic Model Authorstream
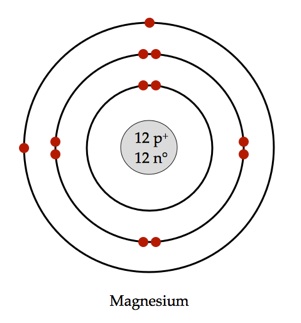



What Would A Bohr Model For Magnesium Look Like Socratic




Question 3 Draw A Sketch Of Bohr S Model Of An Atom With Three Shells Class 9 Science Structure Brainly In




Bohr Model Example Phosphorus Diagram Quizlet




Models Of The Atom The Atom Siyavula



Bohr Atomic Model



What Is In The Middle Of A Bohr Model Quora




Bohr S Atomic Model Ck 12 Foundation
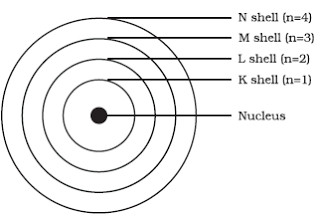



Bohr S Model Of An Atom Class 9 Structure Of An Atom




12 Atomic Models Ideas Bohr Model Atomic Theory Atom



1




What Is An Atom It S A Question Of Physics The Atomic Age Linda Hall Library Kansas City Mo
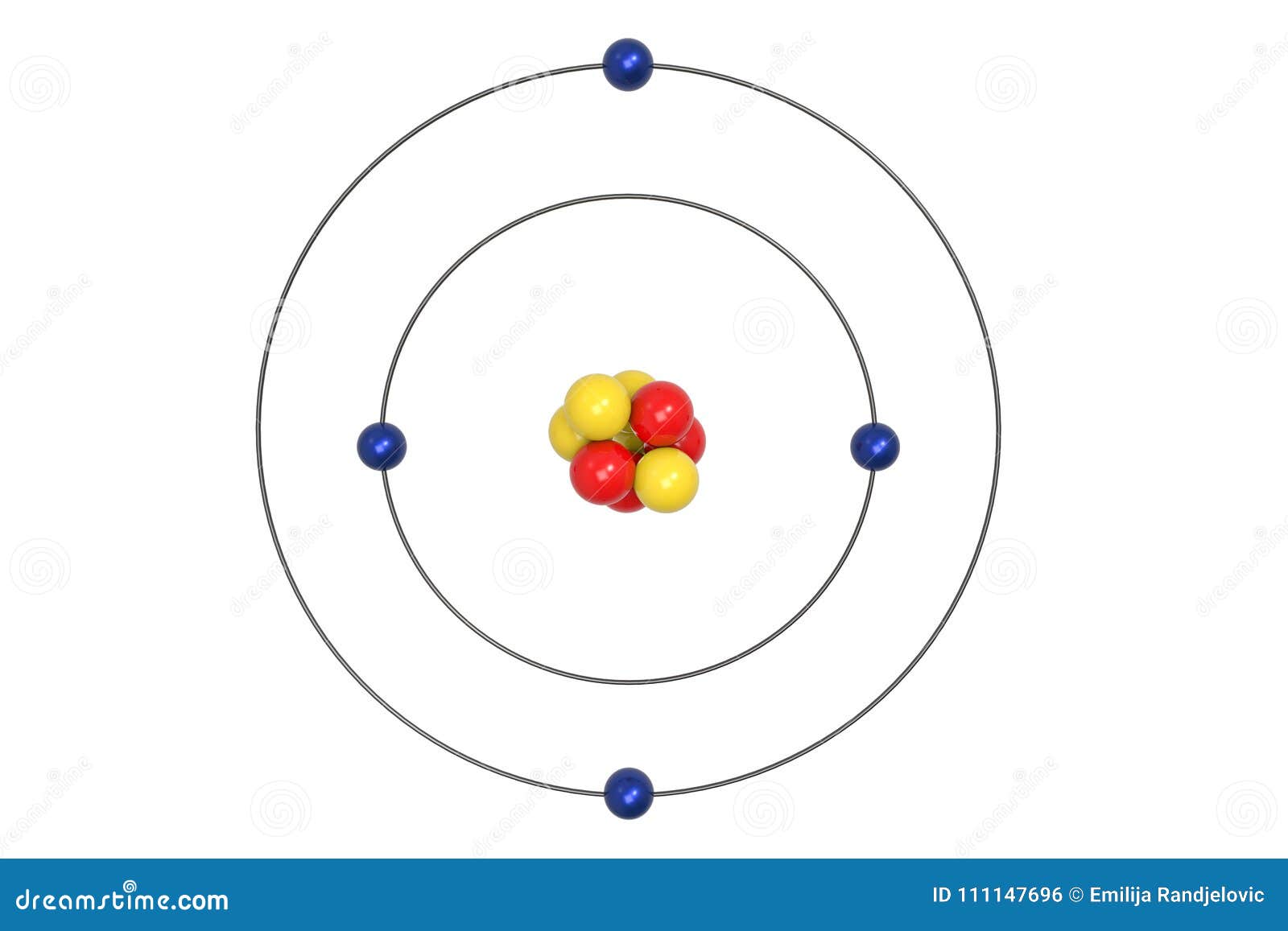



Beryllium Atom Bohr Model With Proton Neutron And Electron Stock Illustration Illustration Of Medicine Ball




Labeled Diagram Of Magnesium Atom Wiring Diagrams Lose




Models Of The Atom The Atom Siyavula




How To Draw The Bohr Rutherford Diagram Of Aluminum Youtube



Bohr Diagrams Of Atoms And Ions Chemistry Libretexts




Image Of An Atom Google Search Bohr Model Atomic Theory Dalton Atomic Model




Bohr Model Description Development Britannica




10 Chem Ideas Bohr Model Physical Science Science Chemistry



Another Look At The Atoms



A Describe The Main Features Of Bohr S Model Of An Atom Draw A Neat And Labelled Diagram Of Energy Levels B Which Of The Following Pairs Are Isotopes And Which Are Isobars
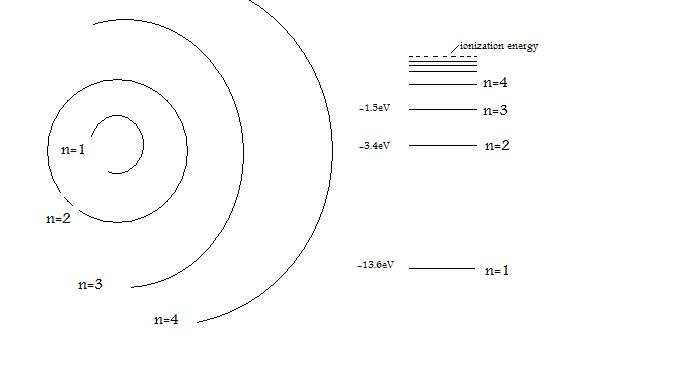



Bohr S Hydrogen Atom Chemistry Libretexts




Dublin Schools Lesson Bohr S Model Of The Atom Whose Atomic Model First Accounted For Defined Energy Levels
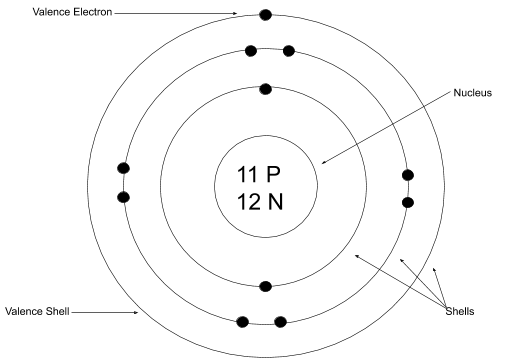



Guide To Bohr Rutherford Models Free Homework Help
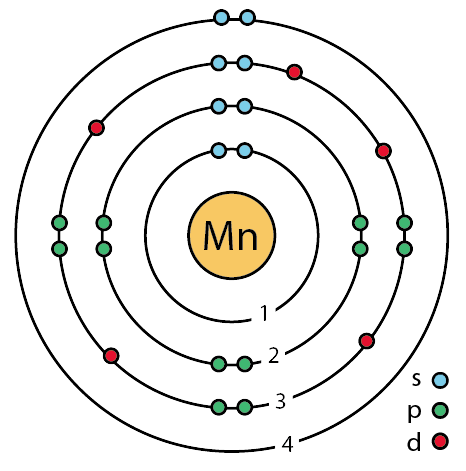



How Do You Draw And Label A Bohr Model For Mn Socratic




Bohr S Model Of An Atom With Postulates And Limitations Byju S



Q Tbn And9gcqcg1zbj Cq9lnv2whtbzywp2s Abxd9 X6d6schpwfg 4go6et Usqp Cau
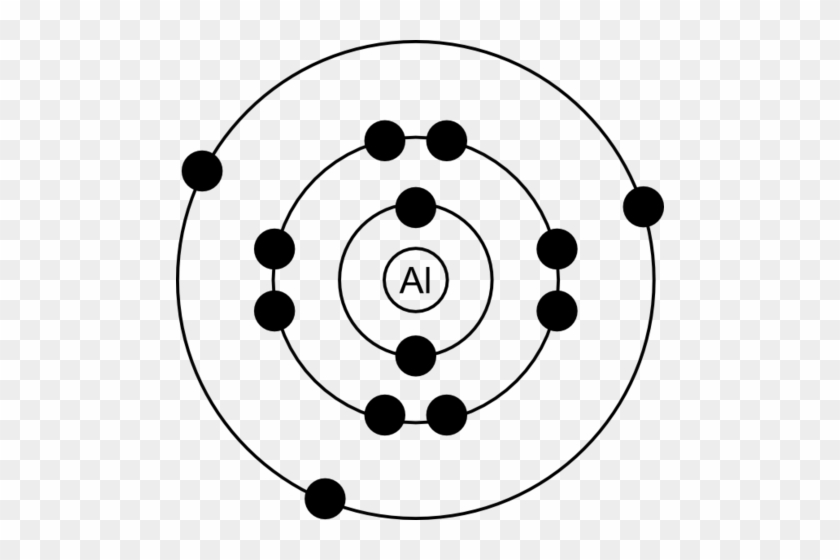



The Onion Atom Bohr Model Of Aluminum Free Transparent Png Clipart Images Download




Bohr Model Description Development Britannica




What Is Bohr S Atomic Model Universe Today




Electron Cloud Atomic Model Ck 12 Foundation



Bohr Model Diagrams Lesson 3 1 Extension Ppt Video Online Download
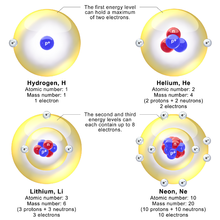



Bohr Model Wikipedia




Snc1p
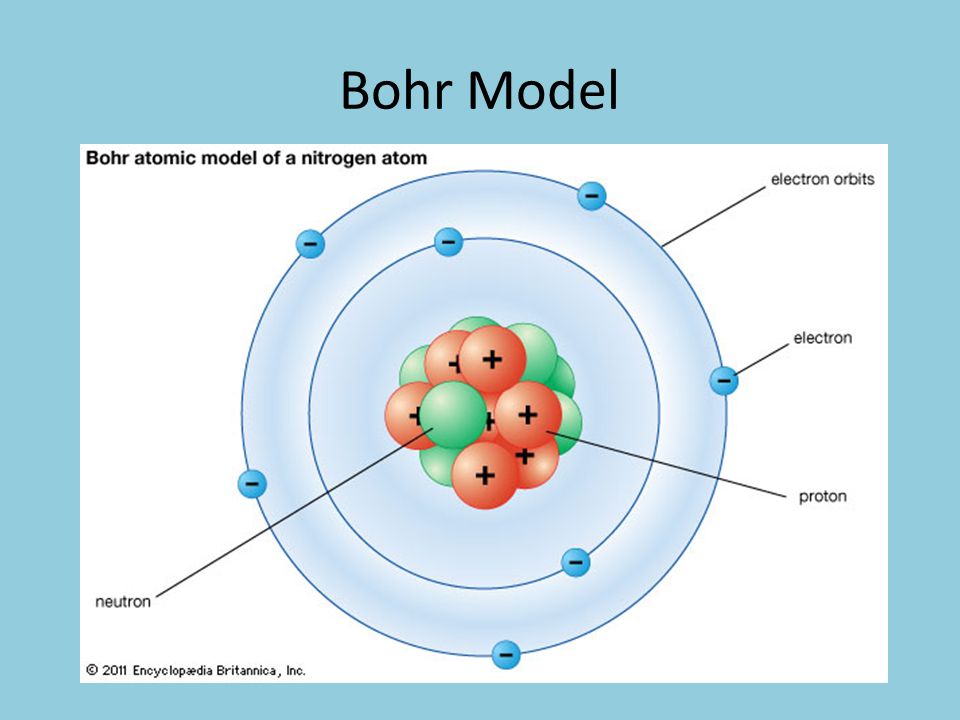



Unit 5 Electrons In Atoms Ppt Video Online Download




High School Chemistry The Bohr Model Wikibooks Open Books For An Open World




Nitrogen Atom Diagram Atom Diagram Atom Model Nitrogen



The Bohr Model Is The Most Accurate Model Of An Atom Fact Or Myth




How To Draw The Bohr Rutherford Diagram Of Carbon Youtube




The Structure Of An Atom Explained With A Labeled Diagram Science Struck



Bohr Atomic Model



What Is Bohr S Atomic Model Quora




Rutherford Model Definition Facts Britannica




Bohr Rutherford Diagram Of A Calcium Atom Source Diagram Calcium Physics
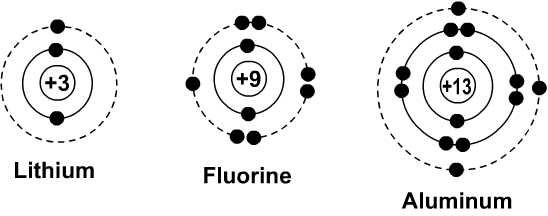



Bohr Diagrams Of Atoms And Ions Chemistry Libretexts
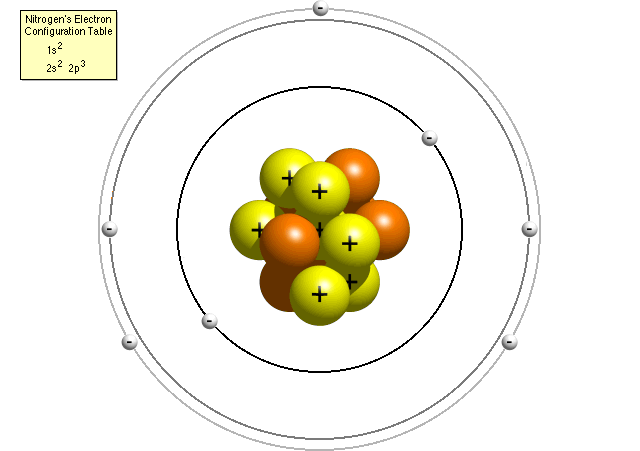



Questions And Answers How Do I Make A Model Of An Atom
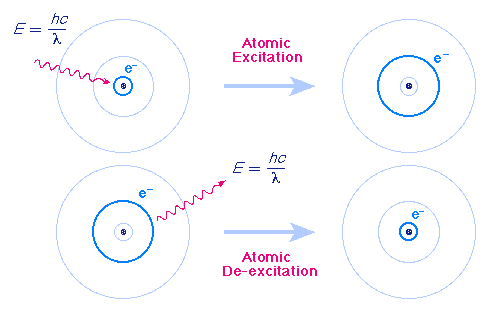



The Bohr Model




Bohr S Model Of Hydrogen Article Khan Academy




Bohr S Atomic Model Ck 12 Foundation




Bohr S Model Of The Hydrogen Atom Diagram Quizlet



2c1 Basic Atomic Structure Jcscience Ie




Bohr S Theory Of The Hydrogen Atom Physics




How Are Different Colored Fireworks Made Sciu




Esen Bohr Model Of The Hydrogen Atom Diagram Quizlet




The Bohr Model Introduction To Chemistry



Why It S Okay To Teach Wrong Ideas In Physics Scientific American Blog Network



Chemical Elements Com Calcium Ca
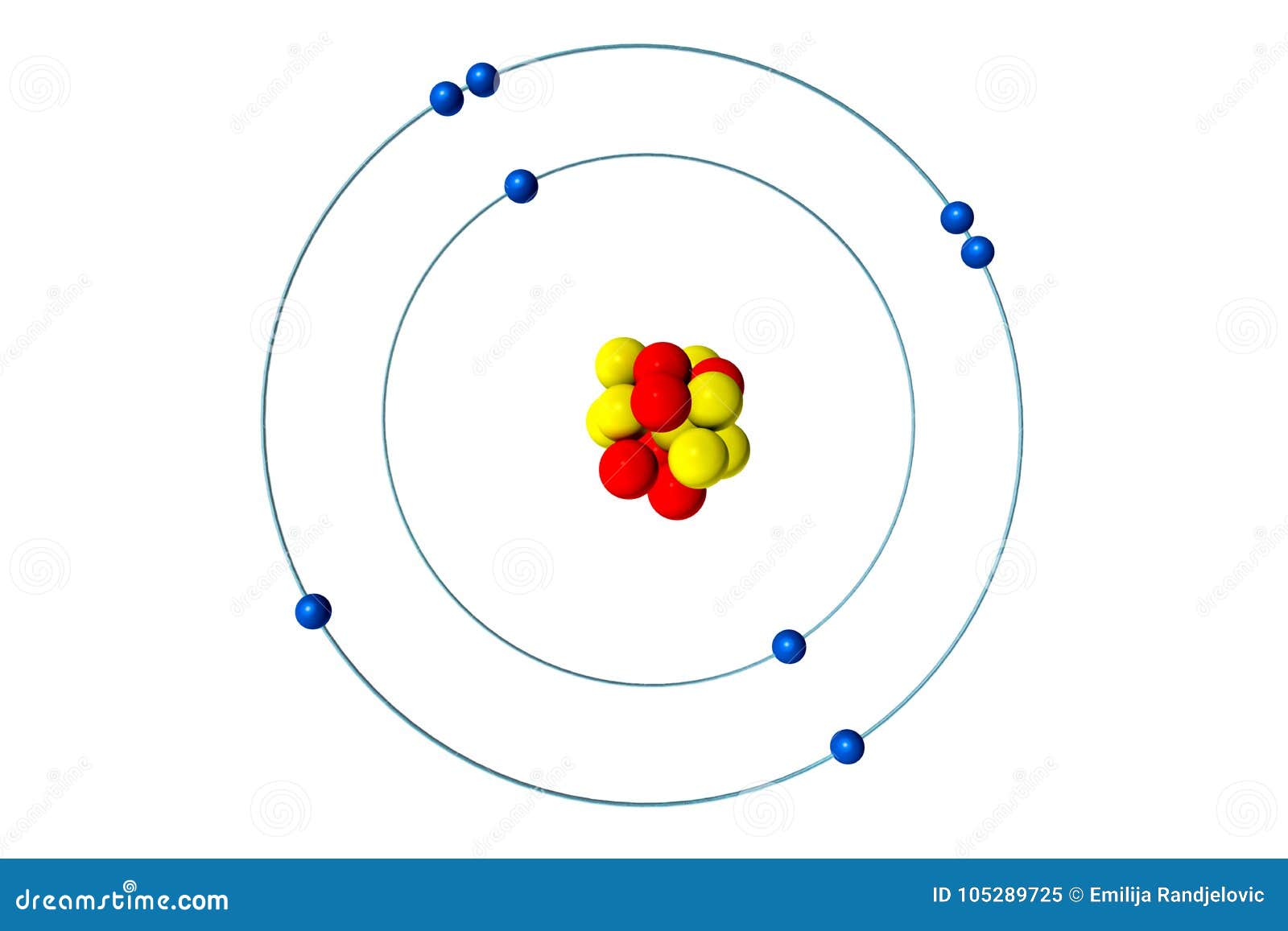



Oxygen Atom With Proton Neutron And Electron 3d Bohr Model Illustration Stock Illustration Illustration Of Chemistry Neutron



Atomic Theory Wikipedia




Bohr Model Wikipedia




Bohr S Model Of An Atom With Postulates And Limitations Byju S




How To Draw The Bohr Rutherford Diagram Of Argon Youtube




Snc1p
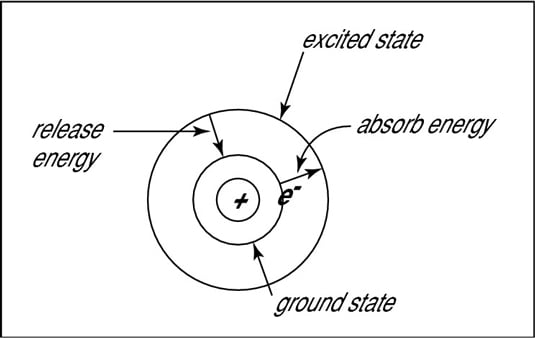



Atomic Structure The Bohr Model Dummies




Atomic Models




2 Shown Below Is A Bohr Model Diagram Representin Chegg Com




The Bohr Model Chemistry For Majors Atoms First



1
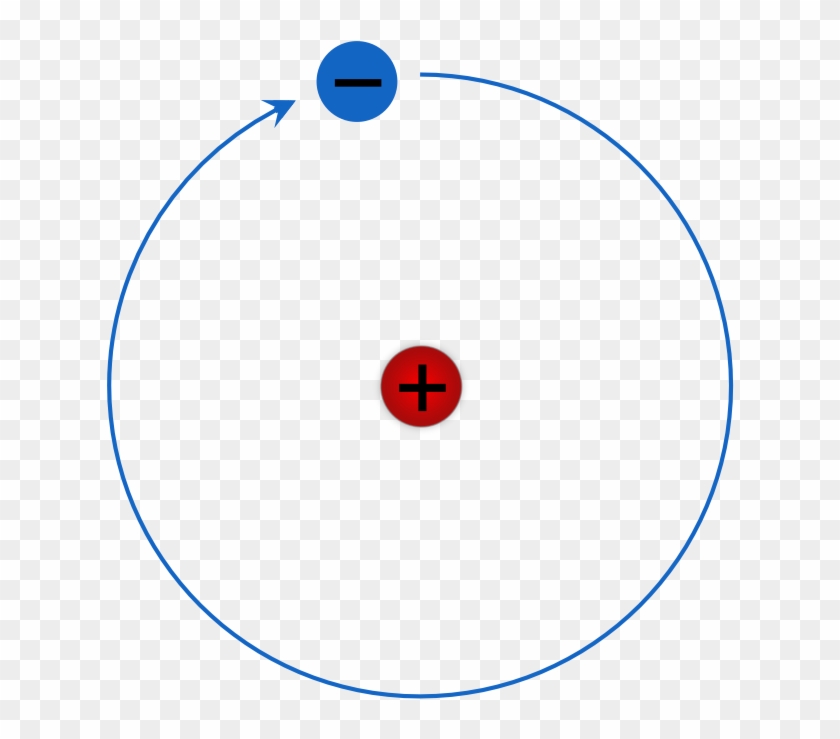



Rutherford Bohr Model Of A Hydrogen Atom Hydrogen Bohr Rutherford Diagram Png Free Transparent Png Clipart Images Download




What Is Bohr S Atomic Model Universe Today




Oxygen Atom Bohr Model With Proton Neutron And Electron 3d Illustration Bohr Model Atom Model Project Atom Model



The Bohr Model
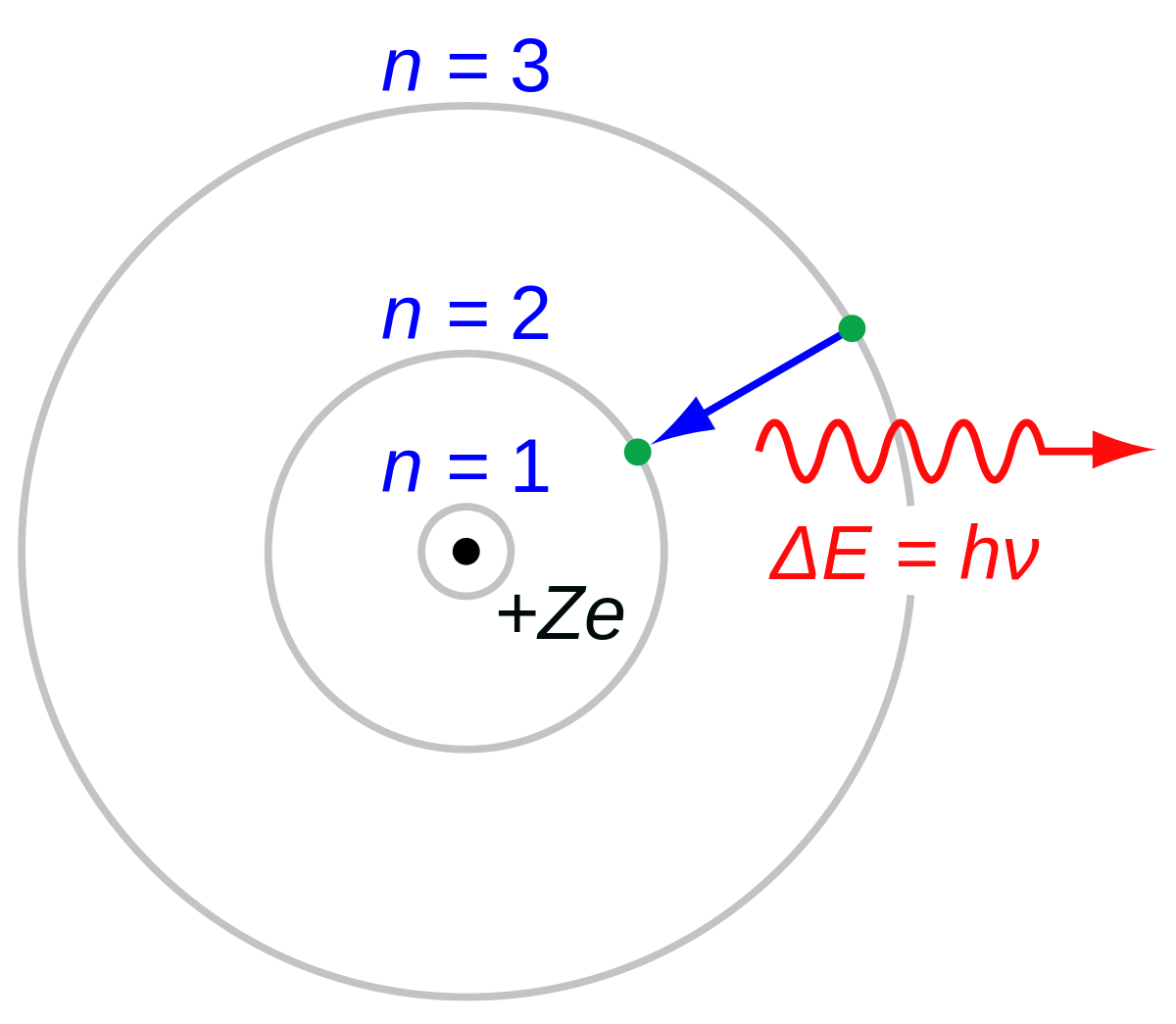



Bohr Model Wikipedia
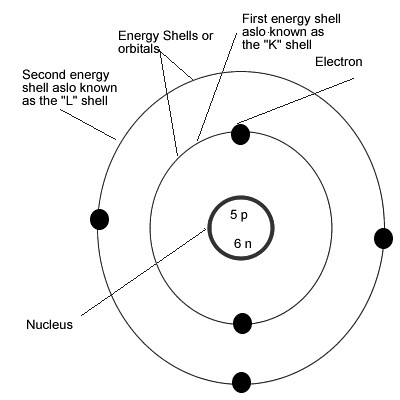



Boron2 The Bohr Rutherford Model Helium Atom Atomic The Flickr
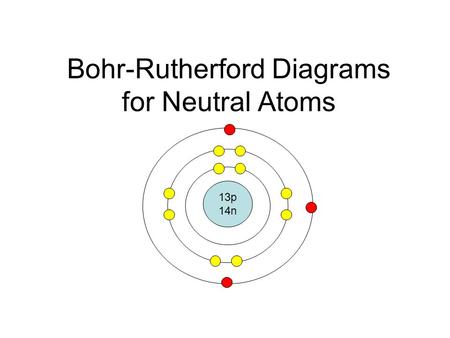



Bohr S Model Of The Atom Ppt Video Online Download
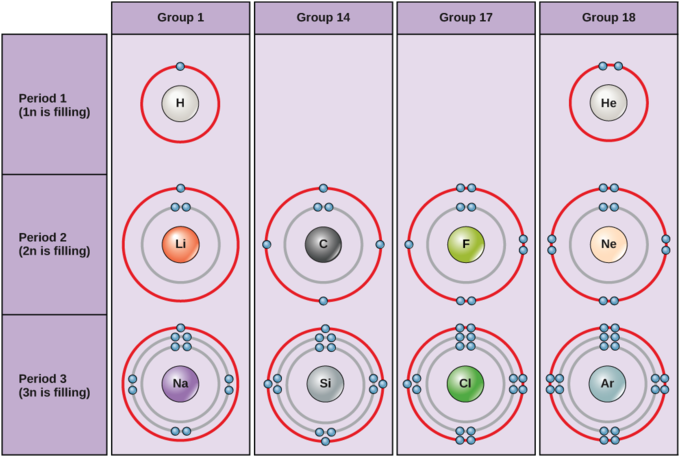



Bohr Diagrams Of Atoms And Ions Chemistry Libretexts


